Molecular environment of water in magmas
- Charles Le Losq
- Feb 22, 2015
- 4 min read
The dynamic (e.g., effusif vs explosive) of volcanic eruption is strongly influenced by how magmas release the dissolved fluids they carry from deep Earth. Water is the main volatile element in magmas, and strongly influences their molecular structure, and hence, their physical properties. Because of that, water concentration in magmas profoundly affects the way volcanoes behave at surface, as we recently showed for Erebus (Antarctica) and Vesuvius (Italy) volcanoes (Le Losq et al., 2015; link to the pdf of the EPSL paper). Interactions between water and magmas are complex, because the way water interacts with the molecular structure of magmas completely drives the solubility of water as well as its effect of the melt properties, such as its viscosity.
At depth in the magma chamber, magmas can contain up to ~6-7 wt% dissolved water. Water resides as molecules in the melt, or is bonded to the melt structure (Fig. 1).

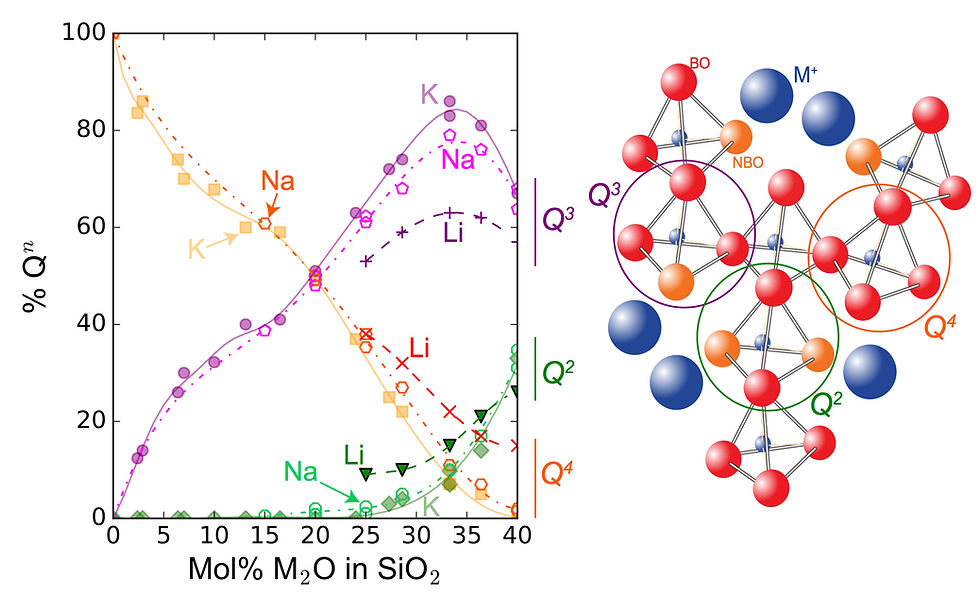
Figure 1: Schematic representation of the effect of solution of water on the silicate structure of a alkali (M+) or alkaline (M2+) oxide (in dark blue) and silicon oxyde binary melt. Si atoms (in dark) are coordinated by four oxygen atoms, forming tetrahedra that are bonded between them by their apical bridging oxygens (BO, in red). Alkali or alkaline-earth atoms break Si-O-Si bonds, forming weak Si-O-M bonds, where oxygens are non bridging (NBO, in green). Water acts as an alkali oxyde, breaking Si-O-Si bonds. This results in the formation of NBO upon solution of water (right part of the figure). However, the reaction does not proceed to completion, and water can also resides as molecules in the network. In this schematic view, we see formation of Si-OH and M-OH groups with solution of water, such processes being confirmed by Silicon NMR and Raman data on silicate glasses (Le Losq et al. submitted to PEPS).
This determines a profound depletion of the melt viscosity, because water tends to break the silicate structure of the melt that ensures its polymerisation, and hence, that drives its viscosity. When the magma rises at surface, the pressure decreases. This results in a decrease of the water solubility in the melt, and water exsolves in bubbles as a gas phase. The molecular structure of the silicate melt changes, because water is not present anymore. As a result, the melt becomes more polymerised, and its viscosity increases. For fluid magmas at eruptive temperature, such as basalts, bubbles can migrate and eventually be released from the magmatic column. Because of that, basaltic magmas often lead to effusive eruptions. However, in viscous magmas, such as for example rhyolitic melts, bubbles are trapped. This can result in the fornation of a magmatic foam in the volcanic conduit. Several parameters influence how this magmatic foam resists to the stresses in the volcanic conduit, but eventually the overpressure of the gases trapped in bubbles as well as the velocity of the flow and its associated strain rate will promote the fragmentation of the magmatic foam. Such a process is crucial in explosive volcanic eruptions.
Temperature strongly influences the way water interacts with the melt structure. The chemical composition of the melt also is important, but its exact effect is much less known. For solving such problem, we started studying with Proton Magic Angle Spinning Nuclear Magnetic Resonance spectroscopy water-bearing glasses that are mixture of alkali oxyde (lithium, sodium or potassium) and silica oxyde. Such composition are the basis of most natural rocks. We show in a new publication in the 100th anniversary issue of American Mineralogist that ionic radius of alkali elements in the melts affects the environment of water. Indeed, the larger the alkali metal is, the stronger the signal from protons in environments with short oxygen-oxygen distances (Fig. 2).

Figure 2: Proton Magic Angle Spinning Nuclear Magnetic Resonance (1H MAS NMR) spectra of the silicate glasses (M is the alkali element) containing 17 mol% dissolved water. The ionic radius of Li is ~82 pm, that of Na ~110 pm and that of K ~146 pm, assuming an oxygen coordinance of 6. Therefore, increasing ionic radius of alkali produces a change in the distribution of protons among several environments, which give different NMR peaks at 1, 3.5, 5, 12 and 16 ppm. Those environments are related to different oxygen-oxygen distances around the protons (top scale). It results that their is a strong change in the oxygen volume around the protons with changing the ionic radius, and hence the ionic field strength, of the alkali metal in the silicate melt. This demonstrates a direct control of the melt chemistry on the environment of dissolved water.
Such effect is related to the partial molar volume of water in the melt, as well as probably to its solubility. Therefore, magmas with different chemistry will present different environments for water, explaining the dependence of water solubility on melt composition. Such results may have important implications for volcanological consideration, because it implies that the magma chemical composition determines the history of its degassing path for instance.
Work published in:



Comments