More on the environment of water in magmas!
- Charles Le Losq
- Jan 28, 2016
- 1 min read
In addition to the work publish in American Mineralogist (see blog post from February 2015), we recently reported on the specific bonding of hydrogen ions in binary alkali silicate melts, some quite simplified version of magmas.
The paper is available in Progress in Earth and Planetary Science, an open-access journal of the Japanese Geoscience Union (JpGU). As the paper is open-access, please feel free to click HERE to download it and have a look at it!
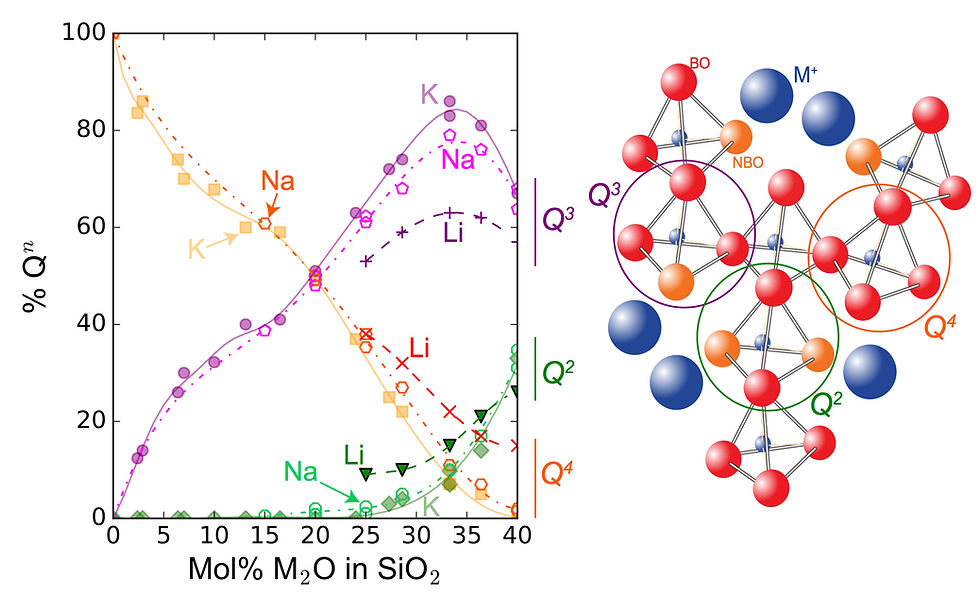
Raman and Nuclear Magnetic Resonance spectroscopy data from hydrous alkali silicate melts allowed us to distinghuish between the hydroxyl groups bonded to Si or alkali ions. Therefore, our last works show that the ionic properties of alkali elements not only control the ratio between unreacted water molecules and hydroxyl groups in melts, but further controls the bonding of the hydroxyl groups with the melt disordered ionic network. Other elements such as alkaline-earth cations probably exert such a control too, explaining why the viscosity of silica-poor basaltic magmas is less depleted by water solution into the melt that the viscosity of silica-rich rhyolitic magmas! Therefore, the magma chemical composition is important to assess how water influences changes in magma viscosity following magma degassing during volcanic eruptions.



Comments